Titration Significant Figures . titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Is titration suitable for sodium nitrate? in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. why is titration used when standardizing a solution? How can i do redox titration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
from www.chegg.com
why is titration used when standardizing a solution? in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. How can i do redox titration. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Is titration suitable for sodium nitrate?
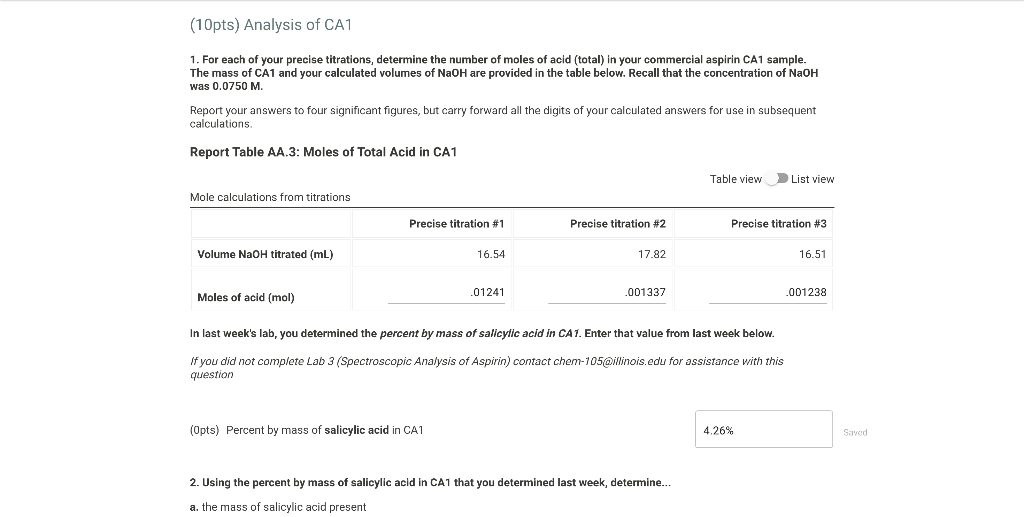
1. For each of your precise titrations, determine the
Titration Significant Figures How can i do redox titration. Is titration suitable for sodium nitrate? in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. How can i do redox titration. titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. why is titration used when standardizing a solution? a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Significant Figures titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. why is titration used when standardizing a solution? a titration is a. Titration Significant Figures.
From www.researchgate.net
Isothermal titrations calorimetry curves of interaction between PA, Ca... Download Scientific Titration Significant Figures in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Is titration suitable for sodium nitrate? a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. a titration is a volumetric technique in which a solution. Titration Significant Figures.
From www.chegg.com
Solved Part A Titration Curves Significant figures /(1 Titration Significant Figures titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. why is titration used when standardizing a solution? a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. Is titration suitable for sodium nitrate? the above equation. Titration Significant Figures.
From www.researchgate.net
Matrix titration of conjugated acceptor beads and streptavidindonor... Download Scientific Titration Significant Figures why is titration used when standardizing a solution? a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. titration is a method. Titration Significant Figures.
From www.numerade.com
SOLVEDThe following titration curve is obtained by titrating 10.00 mL of unknown monoprotic Titration Significant Figures Is titration suitable for sodium nitrate? a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. why is titration used when standardizing a solution? the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. How can. Titration Significant Figures.
From www.numerade.com
SOLVED Match the provided labels to the appropriate point on the titration curve Buffering Titration Significant Figures a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. How can i do redox titration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. why is titration used when. Titration Significant Figures.
From www.numerade.com
SOLVED Text Show a complete calculation string, including units, with the final value reported Titration Significant Figures a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is. Titration Significant Figures.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Significant Figures a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Is titration suitable for sodium nitrate? titration is a method to determine the unknown. Titration Significant Figures.
From skc12chem.blogspot.com
SKC Year 12 Chemistry 2011 Revision Titrations Titration Significant Figures How can i do redox titration. titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a titration, 25.00 cm 3 of 0.200 mol/dm 3. Titration Significant Figures.
From www.vrogue.co
Titration Data Sheetmethod 1 Titration With Buret An vrogue.co Titration Significant Figures a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Is titration suitable for sodium nitrate? How can i do redox titration. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. the above. Titration Significant Figures.
From www.numerade.com
SOLVED ' Name Lab data Record ALL of your measured and calculated data with the correct number Titration Significant Figures a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is. Titration Significant Figures.
From www.slideserve.com
PPT Chapter 15 AcidBase Titrations & pH PowerPoint Presentation ID369037 Titration Significant Figures in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. How can i do redox titration. titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. the above equation works only for neutralizations. Titration Significant Figures.
From www.bartleby.com
Answered Use the titration curve for the weak… bartleby Titration Significant Figures in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Is titration suitable for sodium nitrate? why is titration used when standardizing a. Titration Significant Figures.
From www.chegg.com
1. For each of your precise titrations, determine the Titration Significant Figures in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. How can i do redox titration. the above equation. Titration Significant Figures.
From www.scribd.com
Tips For Titrations PDF Accuracy And Precision Significant Figures Titration Significant Figures the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. How can i do redox titration. a titration is a technique used in chemistry to help. Titration Significant Figures.
From www.chegg.com
Solved Part A Titration Curves Significant figures /(1 Titration Significant Figures titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. How can i do redox titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a volumetric technique in which a solution. Titration Significant Figures.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Significant Figures the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Is titration suitable for sodium nitrate? a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution. Titration Significant Figures.
From www.scribd.com
Booster Chemistry Notes Chapter 1 Laboratory PDF Significant Figures Titration Titration Significant Figures the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. why is titration used when standardizing a solution? in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. titration is a method to. Titration Significant Figures.